I am going to start by talking about carbohydrates. Carbohydrates are one of the main source of energy used by plants and animals to do work. They have the general formula of C*(H*O)* (*=# of atoms/group of atoms). Carbohydrates are considered alcohols due to the -OH groups, anything that has an -OH group is considered an alcohol.
Carbohydrates have many uses to life. They are a source of stored energy that can be released in a way that organisms are able to use. They are used to transport energy in organisms. One of the more interesting aspects of carbohydrates is their ability to be used as structural molecules that give many organisms their shape as well as protection. Carbohydrates can even be used as recognition and signaling molecules that can trigger specific biological responses.
The carbohydrates above are relatively small and are considered to be simple sugars. These simple sugars are called monosaccharides. Monosaccharides can be either five-carbon sugars (pentoses) or six-carbon sugars (hexoses). monosaccharides have the ability to polymerize and become even larger carbohydrates. Polymerization being the ability of certain molecules to become larger molecules such as the disaccharide sucrose. The monomers glucose and fructose can form a glycosidic link that creates the disaccharide sucrose shown bottom in the picture.
Disaccharides,oligosaccharides,and polysaccharides. Oligosaccharides contain several monomers that are all attached with glycosidic linkages. Oligosaccharides can have additional functional groups that give them special properties that a monomer just would not possess.
Polysaccharides are large polymers of monosaccharides connected by glycosidic linkages, and they are not exactly linear. Monomer has several sites that can form glycosidic linkages allowing for branching.
Examples of polysaccharides:
Starches: a family of giant molecules that are all polysaccharides of glucose.
Glycogen: a water-insoluble, highly branched polymer of glucose. Glycogen is used as a the major form of energy storage in mammals. It is produced in the liver and transported to the muscles. Both starch and Glycogen can be readily hydrolyzed into glucose monomers which can be liberated of their energy. The reason why glycogen and starches are broken down the increase amount of osmotic pressure that the individual glucose molecule cause.
Friday, September 26, 2014
Atoms pt 1
Todays studies are going to be on cells. These notes are just going to be little jots of information so bare with me.
Atoms.
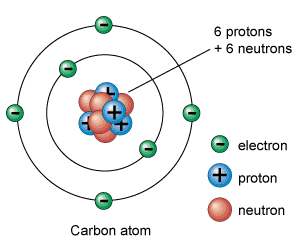
Atoms consist of protons, electrons and neutrons (in some instances they can be without neutrons, like an isotope of hydrogen). Atoms are the building block of the universe and the individual atoms are called elements. Molecules are collections of elements in atomic bonds. Electronegativity has much to do with the strength of the atomic bonds. When the difference between electronegativity is beyond 1.7 those bonds are considered to be ionic and polar. When the difference between electronegativity of the bonds is between .5 and 1.7 they are said to be covalent and polar. Anything below .5 is said to be non polar covalent. Polarity is cause by dipole moment in the atoms, which is when one atom draws more of the electrons to it thus leaving the adjacent atoms lacking in electrons but thriving in the proton department.
Atoms are bonded because of the need for each individual atom to want to have stability. With smaller elements, stability is usually reached when an atom fills all of its valent shell with electrons. These valent electrons are what cause our world to be as it is. Life itself would not be allowed to thrive if is was not for the fact that water, also known as H20, was not a polar molecule. The reason why water is polar is the difference in electronegativity between carbon and oxygen, not only that but the arrowhead shape of the water molecule does not allow its electronegativity difference to be balanced out due to symmetry.
Atoms.
Atoms consist of protons, electrons and neutrons (in some instances they can be without neutrons, like an isotope of hydrogen). Atoms are the building block of the universe and the individual atoms are called elements. Molecules are collections of elements in atomic bonds. Electronegativity has much to do with the strength of the atomic bonds. When the difference between electronegativity is beyond 1.7 those bonds are considered to be ionic and polar. When the difference between electronegativity of the bonds is between .5 and 1.7 they are said to be covalent and polar. Anything below .5 is said to be non polar covalent. Polarity is cause by dipole moment in the atoms, which is when one atom draws more of the electrons to it thus leaving the adjacent atoms lacking in electrons but thriving in the proton department.
Atoms are bonded because of the need for each individual atom to want to have stability. With smaller elements, stability is usually reached when an atom fills all of its valent shell with electrons. These valent electrons are what cause our world to be as it is. Life itself would not be allowed to thrive if is was not for the fact that water, also known as H20, was not a polar molecule. The reason why water is polar is the difference in electronegativity between carbon and oxygen, not only that but the arrowhead shape of the water molecule does not allow its electronegativity difference to be balanced out due to symmetry.
Subscribe to:
Posts (Atom)