Todays studies are going to be on cells. These notes are just going to be little jots of information so bare with me.
Atoms.
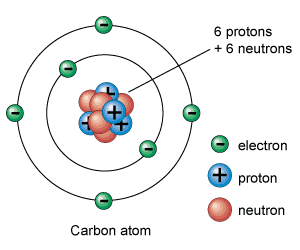
Atoms consist of protons, electrons and neutrons (in some instances they can be without neutrons, like an isotope of hydrogen). Atoms are the building block of the universe and the individual atoms are called elements. Molecules are collections of elements in atomic bonds. Electronegativity has much to do with the strength of the atomic bonds. When the difference between electronegativity is beyond 1.7 those bonds are considered to be ionic and polar. When the difference between electronegativity of the bonds is between .5 and 1.7 they are said to be covalent and polar. Anything below .5 is said to be non polar covalent. Polarity is cause by dipole moment in the atoms, which is when one atom draws more of the electrons to it thus leaving the adjacent atoms lacking in electrons but thriving in the proton department.
Atoms are bonded because of the need for each individual atom to want to have stability. With smaller elements, stability is usually reached when an atom fills all of its valent shell with electrons. These valent electrons are what cause our world to be as it is. Life itself would not be allowed to thrive if is was not for the fact that water, also known as H20, was not a polar molecule. The reason why water is polar is the difference in electronegativity between carbon and oxygen, not only that but the arrowhead shape of the water molecule does not allow its electronegativity difference to be balanced out due to symmetry.
No comments:
Post a Comment